X chromosome inactivation is a fascinating biological process that plays a crucial role in balancing gene expression between males and females. Females possess two X chromosomes, which necessitates the silencing of one to avoid an overload of gene products. This intricate mechanism not only impacts normal cellular function but is also pivotal in understanding various genetic disorders, such as Fragile X Syndrome and Rett Syndrome. Recent advancements, spearheaded by researchers like Jeannie Lee, have shed light on the role of Xist RNA in orchestrating this inactivation, opening doors to potential gene therapy solutions. By unraveling the complexities of X chromosome inactivation, we can explore innovative treatments for these debilitating conditions that stem from mutations on the X chromosome.
The process of silencing one of the two X chromosomes in females, known as X chromosome inactivation, reflects a remarkable genetic balancing act. This strategic mechanism ensures that cells maintain optimal functionality without an excess of gene expression, critical for processes like development and neurological health. With growing insights into this phenomenon, particularly through studies focusing on the Xist RNA molecule, researchers are now poised to address challenges posed by genetic disorders linked to the X chromosome, including symptoms seen in Rett Syndrome and Fragile X Syndrome. Innovations in gene therapy approaches stemming from this research demonstrate significant promise for targeted environmental and genetic interventions. As we delve deeper into the mysteries of X chromosome behavior, we draw closer to therapeutic interventions that could transform the landscape of treatment options for those affected by these genetic conditions.
Understanding X Chromosome Inactivation
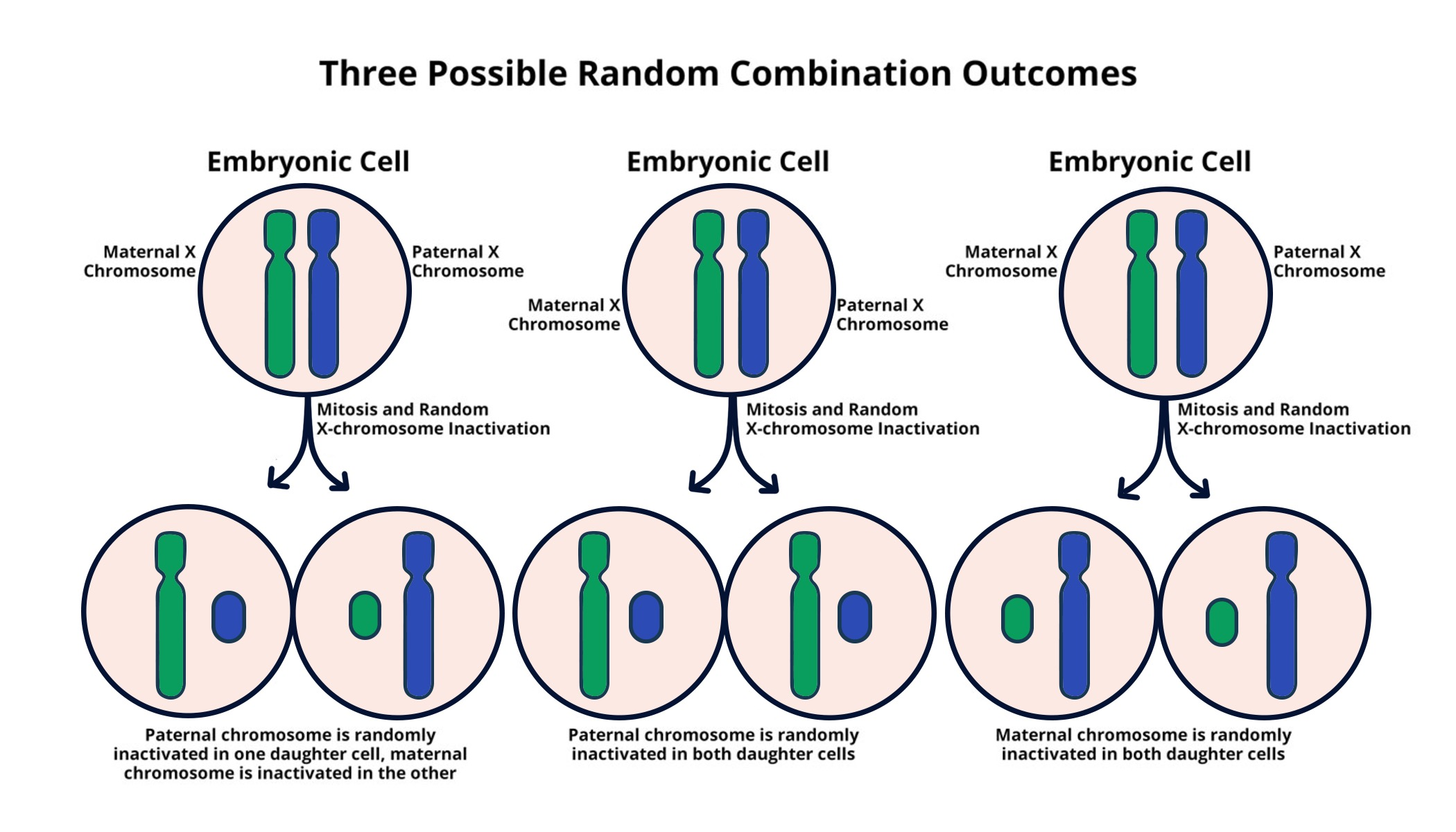
X chromosome inactivation (XCI) is a pivotal process in female mammals, where one of the two X chromosomes is randomly silenced to balance gene dosage between sexes. This phenomenon ensures that females, with two X chromosomes, do not express twice the amount of genes compared to males, who have only one X chromosome. The intricate mechanism of XCI involves the Xist RNA, a long non-coding RNA that plays a crucial role in modifying the chromatin structure of the X chromosome, leading to its inactivation. By coating the chromosome, Xist prevents gene expression, isolating it from the cellular machinery responsible for transcription.
The implications of X chromosome inactivation extend beyond basic biology; they are essential for understanding genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome. Researchers like Jeannie Lee have highlighted how unraveling the mysteries of XCI could pave the way for novel gene therapy approaches. These therapies aim to reactivate silenced gene copies on the inactivated X chromosome, potentially restoring normal function in cells affected by mutations. Thus, studying XCI not only sheds light on female biology but also holds promises for treating debilitating genetic disorders.
The Role of Xist RNA in Gene Therapy
Xist RNA has emerged as a significant player in understanding X chromosome dynamics, particularly concerning potential therapies for genetic conditions such as Fragile X Syndrome. This RNA molecule is responsible for initiating the inactivation of one of the X chromosomes in females, thereby participating in a crucial biological process. Studies indicate that when Xist interacts with the chromatin that surrounds the X chromosome, it alters the structural properties of this chromatin, effectively silencing genes and leading to the isolation of that chromosome from active transcription.
Harnessing Xist RNA’s capabilities opens new avenues for gene therapy. The Lee lab’s pioneering research aims to develop strategies that could ‘unsilence’ the inactivated X chromosome, reactivating beneficial genes that are otherwise dormant due to XCI. This therapeutic strategy has the potential to address genetic disorders by ensuring that the healthy version of genes, such as those commonly mutated in Fragile X Syndrome or Rett Syndrome, become accessible to the cellular machinery again. By employing this approach, researchers hope to correct or alleviate the symptoms associated with these debilitating conditions.
Impacts on Fragile X Syndrome and Rett Syndrome
Fragile X Syndrome and Rett Syndrome are two neurodevelopmental disorders that have strong ties to X chromosome anomalies. Fragile X Syndrome, characterized by intellectual disabilities and developmental delays, is linked to mutations in the FMR1 gene on the X chromosome. Similarly, Rett Syndrome, primarily affecting females, is caused by mutations in the MECP2 gene. The work being done on X chromosome inactivation has significant implications for these conditions, as understanding how to manipulate XCI could directly affect how these disorders are treated. By targeting the effects of Xist RNA, researchers aim to develop gene therapies that may reactivate silenced genes, potentially reversing some effects of these genetic disorders.
The pathway to developing such treatments is reinforced by findings like those from Jeannie Lee’s lab, where researchers are observing how unsilencing techniques can restore functionality to genes that were previously inactivated. This innovative approach holds hope for providing relief to thousands affected by Fragile X Syndrome and Rett Syndrome, as therapies designed to unsilence the X chromosome could enable cells to utilize healthy gene copies more effectively, thereby reducing symptoms and improving quality of life.
Exploring Genetic Disorders and the X Chromosome
Genetic disorders linked to the X chromosome present unique challenges due to the complexities of XCI. Conditions such as Fragile X Syndrome and Rett Syndrome exemplify types of disorders that are inherited in ways that reflect the biology of X chromosome inheritance. As male infants have only one X chromosome, mutations in this chromosome can lead to severe manifestations of these disorders, while females may display a milder spectrum due to XCI. Understanding the processes governing X chromosome inactivation is thus critical in the quest to find therapeutic solutions for these genetic disorders.
The ongoing research exploring X chromosome inactivation not only illuminates the mechanisms at play but also reveals potential targets for interventions. Gene therapy approaches targeting the X chromosome may lead to innovative treatments that specifically address the root causes of conditions like Fragile X and Rett Syndromes. By focusing efforts on manipulating Xist RNA and other factors involved in XCI, researchers are investigating the feasibility of creating novel therapies that could provide significant benefits for individuals affected by these genetic disorders.
The Future of Gene Therapy and XCI Research
The future of gene therapy, particularly in relation to X chromosome inactivation (XCI), is promising, with significant strides being made toward therapeutic applications. With research from institutions like Harvard and the insights provided by leading scientists such as Jeannie Lee, the potential to unlock previously dormant genes on the X chromosome is becoming clearer. By leveraging our understanding of Xist RNA and its role in chromatin modifications, scientists are aiming to develop treatments that could correct genetic disorders caused by X-linked mutations.
As clinical trials loom on the horizon, it is crucial that safety and efficacy studies are conducted to ensure that these therapies can be deployed effectively. The ability to free inactivated X chromosomes could revolutionize the treatment landscape for genetic disorders such as Fragile X Syndrome and Rett Syndrome, potentially changing lives for many patients. The ongoing developments in this area suggest that the techniques for managing XCI will continue to evolve, leading to better outcomes and a deeper understanding of how we can address genetic conditions.
Challenges in Treating X-Linked Genetic Disorders
Despite the advancements in understanding X chromosome inactivation (XCI) and its implications for gene therapy, challenges persist in treating X-linked genetic disorders. The complexity inherent in the biological processes that govern XCI makes it difficult to develop straightforward therapeutic interventions. The selective silencing of the X chromosome in females creates a nuanced landscape, requiring scientists to navigate not only how to reactivate beneficial genes but also how to ensure that healthy genes remain unaffected during treatment.
Further complicating the treatment landscape is the diversity of mutations that can affect X-linked genes. Conditions like Fragile X Syndrome can arise from various mutations within the same gene, creating variability in symptoms and responses to potential therapies. This underscores the need for personalized medical approaches that consider individual genetic profiles. Ongoing research is essential to refine these techniques, make them safe for clinical use, and tailor them to the wide spectrum of genetic disorders associated with the X chromosome.
Mechanisms of X-inactivation in Female Mammals
X-inactivation in female mammals is a fascinating and complex phenomenon that ensures dosage compensation between sexes. The mechanism is primarily driven by Xist RNA, which binds to the X chromosome and initiates a series of changes that lead to gene silencing. Understanding these mechanisms not only contributes to our knowledge of basic biology but also has critical implications for the treatment of genetic disorders. By deciphering the specific processes involved in X chromosome inactivation, researchers can identify targets for therapeutic intervention.
In exploring these mechanisms, it has become apparent that the chromatin structure plays a key role in determining which genes remain active and which become silenced. The gelatinous substance surrounding the chromosomes, likened to Jell-O, provides a unique medium through which gene expression can be finely tuned. Researchers aim to manipulate this environment to facilitate gene activity for those previously inactivated. This understanding of X-inactivation mechanisms offers valuable insights into potential strategies for treating X-linked disorders.
Innovative Approaches to Gene Therapy
Innovative approaches to gene therapy are setting the stage for transformative changes in how we address genetic disorders linked to the X chromosome. With the developments emerging from work at the Lee lab, strategies to target X chromosome inactivation (XCI) are becoming more sophisticated. By focusing on the role of Xist RNA and other key molecules involved in this process, researchers are uncovering potential methods to reactivate silenced genes. Such strategies represent a paradigm shift from traditional gene therapy, which often focuses on replacing missing or dysfunctional genes.
The rise of these innovative techniques has the potential to open doors for treating conditions such as Fragile X Syndrome and Rett Syndrome. Rather than simply delivering new gene copies, the goal is to restore the function of genes that have been rendered inactive due to XCI. This shift not only enhances the feasibility of treatments but also minimizes the risk of adverse effects associated with indiscriminate gene replacement. Continued exploration of these innovative approaches will be critical in the quest to provide effective therapies for X-linked genetic disorders.
Potential for Clinical Trials in XCI Research
As research into X chromosome inactivation (XCI) progresses, the potential for clinical trials grows increasingly tangible. The findings emerging from the Lee lab are underscoring the clinical applicability of understanding XCI mechanisms, particularly concerning genetic disorders like Fragile X Syndrome and Rett Syndrome. Recent developments hint at the possibility of moving from laboratory studies to actual clinical settings, where therapies aimed at unsilencing X-linked genes could be tested for effectiveness and safety.
The prospect of clinical trials represents a critical next step in translating basic research into tangible benefits for patients. With ongoing investment and collaboration among researchers, clinicians, and institutions, there is hope that novel treatment protocols can soon be established. These trials will not only evaluate the efficacy of proposed interventions but also investigate how safely these therapies can be integrated into existing treatment frameworks for genetic disorders, potentially changing the lives of many affected individuals.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to genetic disorders?
X chromosome inactivation is a biological process where one of the two X chromosomes in females is inactivated to prevent overexpression of X-linked genes. This mechanism is crucial in understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome, as mutations on the X chromosome can lead to these conditions while the healthy counterpart may remain silenced. By studying X chromosome inactivation, researchers aim to develop gene therapies that can reactivate these silenced genes.
How does Xist RNA contribute to X chromosome inactivation?
Xist RNA plays a key role in X chromosome inactivation by coating one of the X chromosomes and changing the structure of the surrounding chromatin, or ‘Jell-O’. This process leads to the silencing of that chromosome. Understanding the function of Xist RNA is essential for developing potential therapies for genetic disorders where gene expression must be restored.
Can gene therapy targeting X chromosome inactivation help treat Fragile X Syndrome?
Yes, gene therapy that targets X chromosome inactivation has the potential to treat Fragile X Syndrome. By unsilencing the inactivated X chromosome, healthy copies of genes can be expressed, potentially alleviating the symptoms of this disorder caused by mutations on the X chromosome.
What is the significance of studies on X chromosome inactivation for Rett Syndrome patients?
Studies on X chromosome inactivation are significant for Rett Syndrome patients as they explore ways to reactivate the silenced healthy X chromosome. This mechanism could enable the expression of functional genes that are muted due to X chromosome inactivation, representing a promising direction for effective therapies.
How do researchers hope to un-silence X-linked genes in clinical applications?
Researchers like Jeannie Lee are developing methods to un-silence X-linked genes by manipulating the Jell-O-like substance surrounding chromosomes and utilizing Xist RNA. These approaches are being refined for clinical applications, particularly for disorders like Fragile X Syndrome and Rett Syndrome, with hopes of initiating clinical trials in the near future.
What challenges are associated with understanding X chromosome inactivation and its potential therapies?
Understanding X chromosome inactivation poses challenges due to its complex biological mechanisms. While research shows potential therapies for genetic disorders like Fragile X Syndrome, ensuring that only mutated genes are targeted without affecting healthy genes remains an area needing further investigation.
How does X chromosome inactivation affect males with X-linked genetic disorders?
Although males have only one X chromosome and do not undergo X chromosome inactivation, they can still be affected by mutations on that X chromosome. For instance, in diseases like Fragile X Syndrome, targeted therapies may potentially reactivate beneficial gene expressions, even in male patients.
What are the prospects for future research on X chromosome inactivation and gene therapy?
The future of research on X chromosome inactivation looks promising, with potential for developing gene therapies for conditions like Fragile X and Rett Syndromes. Current studies aim to optimize methods for unsilencing genes on the inactivated X chromosome and proceed to safety studies and clinical trials in upcoming years.
Why is the study of X chromosome inactivation important for genetic disease treatments?
Studying X chromosome inactivation is crucial because it opens avenues for therapies aimed at genetic disorders linked to X-linked mutations. By understanding the mechanisms of inactivation, scientists can devise strategies to restore the function of mutated genes, potentially editing the landscape of treatment options for affected individuals.
| Key Point | Details |
|---|---|
| X Chromosome Challenge | Females have two X chromosomes while males have one, creating a need for X chromosome inactivation in females. |
| Role of Xist Gene | The Xist gene produces an RNA molecule that plays a critical role in the inactivation process by modifying the surrounding ‘Jell-O’ substance. |
| Chromosomal Jell-O | The gelatinous substance around chromosomes helps prevent tangling and plays a crucial role in X chromosome inactivation. |
| Potential Therapies | Research aims to unsilence inactivated X chromosomes to treat disorders like Fragile X Syndrome and Rett Syndrome. |
| Implications for Males | Methods developed could also benefit males, particularly for treating X-linked mutations. |
| Research Support | Support from National Institutes of Health has been pivotal to advancing knowledge of X chromosome inactivation. |
Summary
X chromosome inactivation is a crucial process that allows females to manage the presence of two X chromosomes without the need for excess gene expression. Through recent research led by Jeannie T. Lee, scientists have begun to understand the molecular mechanisms behind this process, involving the Xist gene and a gelatinous substance that modifies the properties of chromosome packaging. This breakthrough not only illuminates a long-standing question in cell biology but also opens the door to potential therapies for genetic disorders linked to mutations in the X chromosome, such as Fragile X and Rett syndromes. The implications of these findings could pave the way for innovative treatments that could ultimately lead to cures for affected individuals.