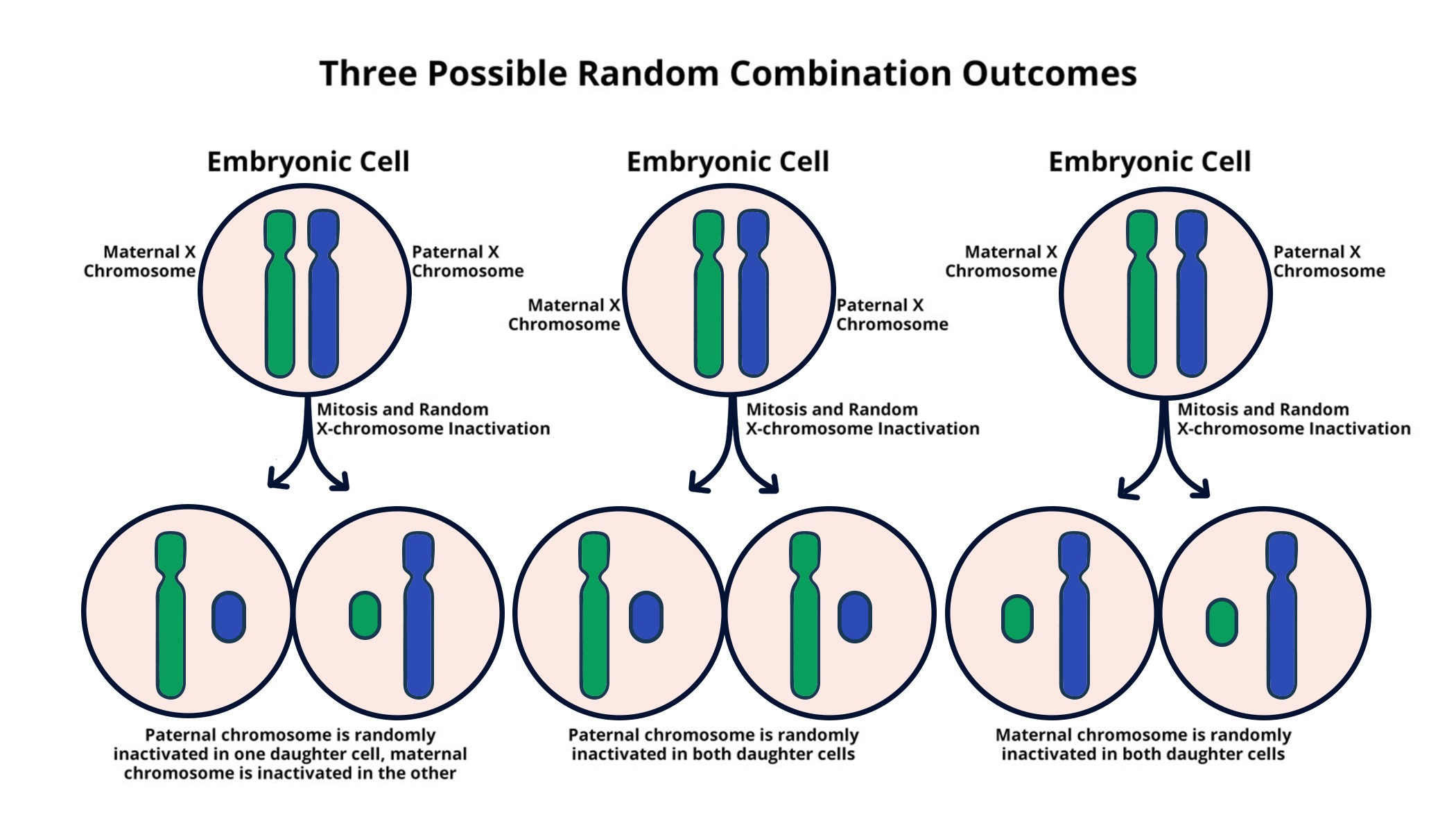
X chromosome inactivation is a fascinating process that plays a crucial role in the biology of female mammals. In contrast to their male counterparts, females possess two X chromosomes, yet only one is actively expressed while the other undergoes chromosomal silencing. This unique mechanism is vital for maintaining genetic balance and preventing an overload of X-linked genes. The understanding of X chromosome inactivation has significant implications for understanding genetic disorders such as Fragile X Syndrome and Rett Syndrome, which arise from mutations on the X chromosome. As researchers uncover the intricacies of this process, it opens the door to potential new therapies for individuals affected by these X-linked disorders.
The phenomenon of inactivating one of the X chromosomes in females is critical for ensuring proper gene dosage and function. This process, often referred to as dosage compensation, allows females to balance the expression of X-linked genes, which is particularly important given their double X chromosome inheritance. By silencing one X chromosome, cells prevent the overexpression that could lead to complications in processes like development and cellular function. This intricate balance not only sheds light on chromosomal behavior but also offers insights into serious genetic conditions, including Fragile X Syndrome and Rett Syndrome, revealing the interconnectedness of chromosomal silencing and genetic health.
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a crucial biological process that mitigates the gene dosage imbalance between males and females. In females, having two X chromosomes means that one must be inactivated to ensure that cells have only one functional copy of the genes located on the X chromosome, preventing an excess of gene expression that could be detrimental. This sophisticated mechanism is essential for the proper functioning of cellular activities and is a significant area of study for geneticists and cell biologists alike.
The process of X-inactivation is orchestrated by various molecular players, notably the long non-coding RNA known as Xist. Upon activation, Xist spreads across the X chromosome and induces changes in chromosomal structure, leading to chromosomal silencing. Understanding how Xist interacts with chromatin and other cellular components will shed light on many genetic disorders caused by X-linked mutations, including Fragile X Syndrome and Rett Syndrome, which affect many individuals worldwide.
The Role of Jell-O-like Substances in Chromosomal Silencing
Recent research highlights the role of a gelatinous substance, described as Jell-O, that envelops chromosomes and plays a critical role in chromosomal silencing, particularly during X chromosome inactivation. This substance serves to separate chromosomes, preventing them from tangling and interfering with one another during cell division. The interaction between Xist and this Jell-O-like material is fundamental in understanding how DNA is packaged and accessed within the nucleus, which is essential for gene regulation.
As Xist infiltrates this gelatinous matrix, it alters the biophysical properties of the substance, enhancing its flexibility and allowing other regulatory molecules to access regions of the X chromosome. This dynamic interaction not only facilitates the inactivation process but also opens avenues for therapeutic interventions aimed at diseases like Fragile X Syndrome and Rett Syndrome. By manipulating this Jell-O-like coating, scientists hope to develop treatments that can reactivate inactivated genes, providing hope for individuals affected by these genetic disorders.
Potential Therapies for Fragile X and Rett Syndromes
The insights gained from studies on X chromosome inactivation have paved the way for promising therapeutic strategies targeting Fragile X Syndrome and Rett Syndrome. Researchers are applying their understanding of Xist and its interactions with Jell-O-like substances to devise methods that can unsilence inactivated X-linked genes. This approach not only holds the promise of restoring function to genes that carry mutations but also represents a novel class of treatments for genetic disorders that were previously deemed irreparable.
By focusing on the potential to reactivate the healthy copy of mutated genes in individuals, these therapies could change the lives of many affected patients. As scientists like Jeannie Lee continue to optimize these methods and conduct safety studies, the prospect of moving these therapies into clinical trials is becoming increasingly realistic. Success in these endeavors could lead to groundbreaking advances in treating intellectual disabilities and neurodevelopmental disorders linked to X-linked gene mutations.
Challenges in Gene Reactivation Strategies
While the potential benefits of reactivating inactivated genes are significant, there are challenges inherent in the gene reactivation strategies being explored. One major concern is the specificity of the treatments; the goal is to ensure that only the mutated genes are reactivated without disturbing the normal function of other genes on the X chromosome. This requires a precise understanding of how cells regulate gene activity to avoid unintended consequences in gene expression.
Moreover, the possibility that freeing inactivated X chromosomes may lead to off-target effects must be studied thoroughly before clinical application. Scientists must navigate the complexities of chromosomal dynamics, understanding how cells utilize their capacity for gene expression while minimizing risks associated with gene therapy. Thus, ongoing research must address these hurdles to harness the full potential of X chromosome inactivation in treating genetic disorders.
Exploring Genetic Disorders Linked to X Chromosomes
X-linked genetic disorders, such as Fragile X Syndrome and Rett Syndrome, have unique patterns of inheritance and manifestation in affected individuals. The complexity of these disorders arises not only from the mutations present on the X chromosome but also from the intricate regulatory mechanisms that govern gene expression. Understanding the underlying biology of these X-linked genes unlocks potential therapeutic pathways that can benefit those affected.
Further exploration of genetic disorders linked to X chromosomes provides valuable insights into their pathophysiology. Research into chromosomal silencing and the role of Xist, along with advances in gene editing technologies, can lead to innovative treatment strategies that address the root causes of these conditions. Continued investigation in this field is essential for developing effective therapies that can significantly improve the quality of life for individuals living with X-linked genetic disorders.
Recent Breakthroughs in Chromosomal Research
Recent breakthroughs in the understanding of X chromosome inactivation represent a significant advancement in the field of genetics. Researchers at leading institutions have unveiled mechanisms that elucidate how XCI operates at the molecular level, providing clarity on processes that have been a mystery for decades. The discovery of the Jell-O-like substance surrounding chromosomes and its role in chromosomal silencing is a prime example of how innovative research can lead to potential therapeutic applications.
These findings pave the way for novel approaches in treating genetic disorders that stem from mutations on the X chromosome. With advancements in lab techniques and a deeper understanding of chromatin biology, the potential to discover effective treatments for Fragile X Syndrome and Rett Syndrome is more promising than ever. Scientific endeavors in this area underscore the critical importance of continued funding and research to unlock further discoveries that can lead to breakthroughs in medical science.
The Future of Genetic Therapy Research
The future of genetic therapy research is poised for unprecedented growth and innovation, especially in the context of X-linked disorders. With the advancements in understanding chromosomal silencing mechanisms, researchers are now equipped to develop targeted therapies that can reactivate silenced genes. As treatments for conditions like Fragile X Syndrome and Rett Syndrome inch closer to clinical trials, anticipation builds for breakthroughs that could reshape patient outcomes.
Furthermore, the intersection of molecular biology and technology, such as CRISPR and other gene editing techniques, presents exciting opportunities for genetic therapies. Future research will focus on refining these methodologies to ensure precision and safety in treating genetic disorders. As we continue to uncover the complexities of gene regulation and expression, the potential to address previously untreatable conditions becomes a realizable goal for the medical community.
The Impact of X-linked Gene Mutations
X-linked gene mutations can have profound effects on individuals and families, affecting not only health but also quality of life. Disorders such as Fragile X Syndrome and Rett Syndrome are linked to mutations that disrupt normal cellular functions, leading to a range of symptoms from developmental delays to cognitive impairment. Understanding these mutations and their impacts is vital for developing effective interventions and support systems for affected individuals.
The effects of X-linked gene mutations extend beyond clinical symptoms, influencing the psychosocial aspects of living with a genetic disorder. Families often navigate complex emotional and financial challenges, and the unpredictability of disease progression can be overwhelming. By advancing our understanding of X chromosome biology and the implications of these mutations, researchers and healthcare providers can better support patients and families in their journeys.
Innovations in Genetic Research Methodologies
Innovations in genetic research methodologies are revolutionizing the way scientists study X-linked disorders. The advent of advanced imaging techniques and high-throughput sequencing technologies has enabled researchers to dissect the intricate processes involved in X chromosome inactivation. These new methodologies not only enhance our understanding of fundamental biological mechanisms but also facilitate the identification of potential therapeutic targets within X-linked genes.
As the landscape of genetic research evolves, collaborations between molecular biologists, clinicians, and geneticists are becoming increasingly important. The integration of interdisciplinary approaches will likely yield groundbreaking discoveries that accelerate the development of treatments for genetic disorders. Empowered by new tools and collaborative efforts, the scientific community is well-positioned to make significant strides in the field of genetics, particularly in addressing the challenges posed by X-linked gene mutations.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation is a biological process where one of the two X chromosomes in female mammals is randomly silenced to prevent an overload of gene expression. This process is crucial in genetic disorders such as Fragile X Syndrome and Rett Syndrome, as it helps to balance gene dosage between males, who have one X chromosome, and females, who have two. Understanding this mechanism can lead to potential treatments by reactivating the silenced genes.
How does X chromosome inactivation contribute to Fragile X Syndrome?
X chromosome inactivation plays a significant role in Fragile X Syndrome, a genetic disorder linked to mutations on the X chromosome. In females carrying the mutation, the affected X chromosome can be silenced while the other, functioning X chromosome may remain active. This dynamic underlies the varying expression of symptoms in females, but males with this mutation often exhibit more severe symptoms due to having only one X chromosome.
What are the mechanisms involved in chromosomal silencing during X chromosome inactivation?
Chromosomal silencing during X chromosome inactivation involves the action of the Xist RNA molecule, which coats the X chromosome, altering the biophysical properties of surrounding chromosomal material, often referred to as ‘Jell-O’. This coating process makes the chromosome inactive by enabling other molecules to facilitate its silencing, thus preventing gene expression.
Can understanding X chromosome inactivation lead to treatments for Rett Syndrome or Fragile X Syndrome?
Yes, research into X chromosome inactivation has opened pathways to potential treatments for Rett Syndrome and Fragile X Syndrome. By developing methods to unsilence the inactivated X chromosome, scientists hope to restore function to mutated genes, which could alleviate symptoms associated with these genetic disorders.
What role does Xist play in X chromosome inactivation and how is it related to genetic disorders?
Xist, or X-inactive specific transcript, is critical in X chromosome inactivation as it initiates the silencing of one X chromosome. Its interaction with the chromosomal ‘Jell-O’ modifies the structure to facilitate inactivation. This process is especially relevant in genetic disorders like Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome can lead to significant health issues.
Are there techniques being developed to reverse X chromosome inactivation for therapeutic purposes?
Yes, researchers, particularly in Jeannie Lee’s lab, are developing techniques to reverse X chromosome inactivation. These approaches aim to reactivate silenced X-linked genes related to genetic disorders such as Fragile X Syndrome and Rett Syndrome, potentially providing new therapeutic options for those affected.
What challenges remain in understanding X chromosome inactivation and its effects on diseases?
Despite advancements, challenges remain in fully understanding X chromosome inactivation. For instance, while some mutated genes can be restored upon unsilencing, it remains unclear why healthy genes on the X chromosome are less affected. Further research is needed to clarify these mechanisms and enhance therapeutic strategies.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | In females, one of the two X chromosomes is inactivated to prevent gene overexpression. |
| Role of Jeannie Lee’s Research | Lee’s lab has been pivotal in understanding X chromosome inactivation mechanisms. |
| Mechanism of Inactivation | Inactivation involves Xist RNA, which alters the biophysical properties of a gelatinous substance surrounding chromosomes. |
| Potential Treatments | Research aims to develop therapies for Fragile X and Rett syndromes by unsilencing mutated genes. |
| Clinical Trials | Optimizing approaches and conducting safety studies before potential clinical trials. |
| Remaining Mysteries | It’s unclear why freeing inactivated chromosomes mostly restores function to mutated genes only. |
| Historical Context | Research on X chromosome inactivation has spanned decades, supported by NIH funding. |
Summary
X chromosome inactivation is a crucial biological process whereby females deactivate one X chromosome to maintain genetic balance compared to males. Recent studies from Jeannie T. Lee’s lab have shed light on how this intricate process works, with implications for treating genetic disorders linked to the X chromosome. Understanding X chromosome inactivation is not only essential for basic biology but also holds promise for future clinical applications, potentially alleviating the burden of conditions such as Fragile X Syndrome and Rett Syndrome.